
How did JJ Thomson know the electron was negative? Can a scientist replicate the findings of another scientist?Ī scientist can only replicate a scientific investigation if he or she watched someone else perform it. This theory further helped physicists in understanding the structure of an atom. Thomson concluded that rays were and are basically negatively charged particles present or moving around in a set of a positive charge. What was the conclusion of the cathode ray experiment?Ĭonclusion.
#Jj thomson cathode ray experiment without word full#
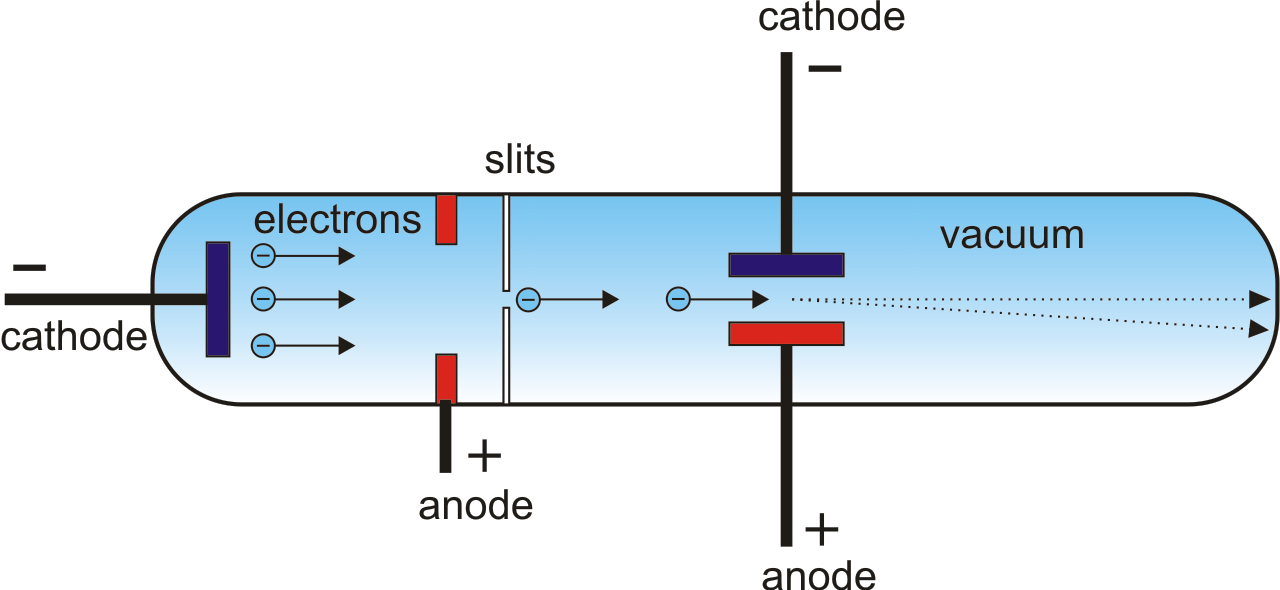
Thomson, in full Sir Joseph John Thomson, (born December 18, 1856, Cheetham Hill, near Manchester, England-died August 30, 1940, Cambridge, Cambridgeshire), English physicist who helped revolutionize the knowledge of atomic structure by his discovery of the electron (1897). When did JJ Thomson discovered the electron? He observed that cathode rays were deflected by a magnetic field in the same manner as a wire carrying an electric current, which was known to be negatively charged. In order to determine if the cathode ray consisted of charged particles, Thomson used magnets and charged plates to deflect the cathode ray. How did Thomson know the charge of a particle discovered was negative? This indicated that the cathode ray was composed of negatively-charged particles. The cathode ray was deflected away from the negatively-charged electric plate and towards the positively-charged plate. To test the properties of the particles, Thomson placed two oppositely-charged electric plates around the cathode ray. How did Thomson’s cathode ray experiment work? Kaufmann concluded that the hypothesis that cathode rays were particles was inconsistent with this result. Thomson found that cathode rays always had the same e/m ratio, no matter what metals were used for the cathodes and no matter what gas was used in the tubes. What was the significance of the fact that Thomson saw the same cathode rays for any gas with which he filled the tube? Finally, in the third experiment, he found that these particles were smaller than an atom. He deduced that the cathode rays were made up of negatively-charged particles. In Thomson’s first experiment, he discovered that cathode rays and the charge they deposited were intrinsically linked together. The negative electrons represented the raisins in the pudding and the dough contained the positive charge.

He demonstrated that cathode rays were negatively charged. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. What did Thomson determine the charge of an electron to be positive or negative? 6 What does Hempel mean by the phenomenalist account?.5 Can a scientist replicate the findings of another scientist?.4 When did JJ Thomson discovered the electron?.3 How did Thomson know the charge of a particle discovered was negative?.2 What happened in Thomson’s experiment?.

1 What did Thomson determine the charge of an electron to be positive or negative?.This was the beginning of further understanding leading to the atomic theory and structure that we know today.Ĭathode ray tube is a tube that contains a small amount of gasīetween two metallic plates. He won the 1906 Nobel prize for the discovery of electrons. He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a British physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, J.


 0 kommentar(er)
0 kommentar(er)
